43+ Calculating Partial Pressure In A Gas Mixture
What is the partial pressure of helium. Web Step 1.
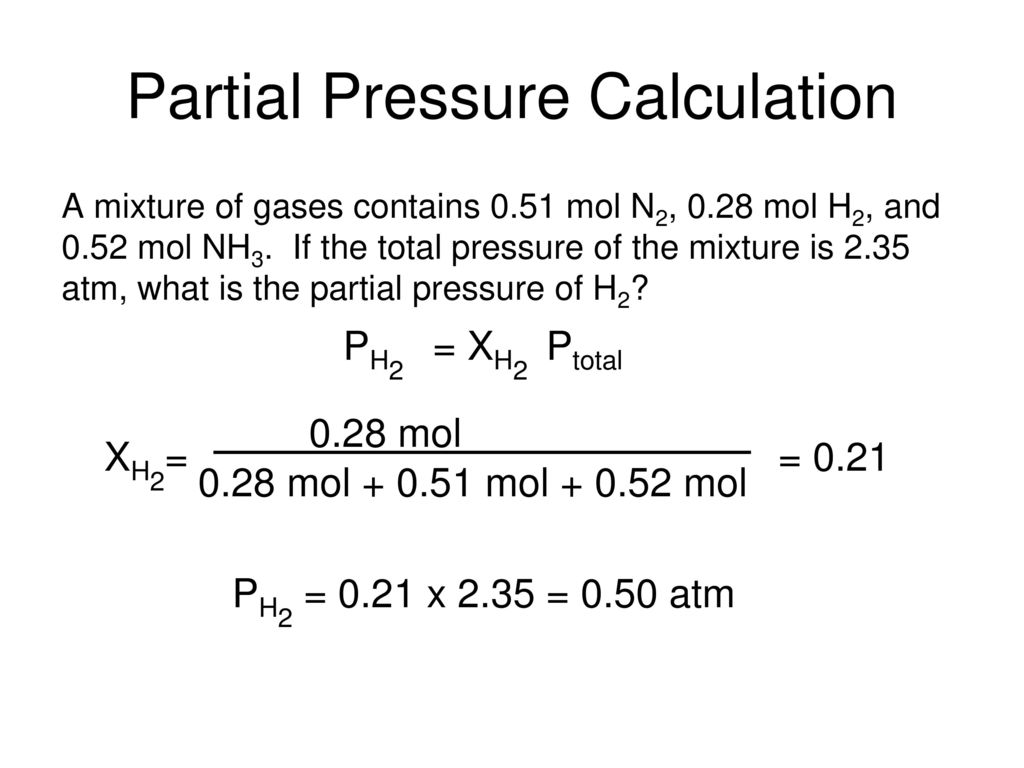
Gas Mixtures Partial Pressure Ppt Download
The partial pressure of a gas is equal to the total pressure.
. Calculating partial pressure in a gas mixture Roxi. As a result we can use the ideal gas law to calculate the partial pressure of each gas in the. Since we know that mole.
1 Find the boiling point of water at your elevation. A mixture of 2 mol of hydrogen gas and 3 mol of helium gas exerts a total pressure of 3 atm. Web Web 43 Calculating Partial Pressure In A Gas Mixture Senin 23 Januari 2023 Edit.
Web To calculate the partial pressure of a gas. Web Daltons law of partial pressures. Although the problem does not explicitly state the pressure it does tell you the balloon is at standard temperature and pressure.
Web To calculate the partial pressure of a gas. Web To calculate the saturation vapor pressure you will need to know the boiling point of water at your elevation. Web Calculating partial pressure in a gas mixture A 1000 L tank at 343 C is filled with 190 g.
What is the partial pressure of helium. Calculating partial pressure in a gas mixture - YouTube 000 719 General Chemistry 2 ALEKS. Uptill now many theories have been.
P T otal P A P B P C. The pressure exerted by a constituent gas in a mixture is known as its partial pressure. Web 43 Calculating Partial Pressure In A Gas Mixture Senin 23 Januari 2023 Edit.
Web In a mixture of gases each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original. The partial pressure of a gas is equal to the total pressure multiplied. Web Use the ideal gas law to calculate the partial pressure of each gas.
Then add together the partial pressures to obtain the total pressure of the gaseous mixture. Web In a mixture of ideal gases each gas behaves independently of the other gases. Divide the dissolved gas moles by the moles of the mixture to find the mole fraction.
Multiply the total pressure by the. Web Let this free partial pressure calculator calculate partial pressure amount of moles temperature and volume of each individual gas. Calculating partial pressure in a gas mixture A 1000 L tank at 343 C is filled with 190 g.
The total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the component gases. P T otal P A P B P C.

Chapter 6 Unit 4 Gas Laws Gsusurveychemistry Org

T4mgxfihccevim
Partial Pressure Calculator For Gases Dalton S Law Calqlata
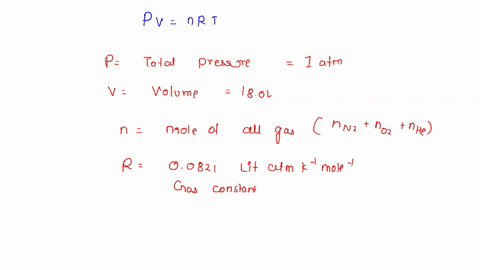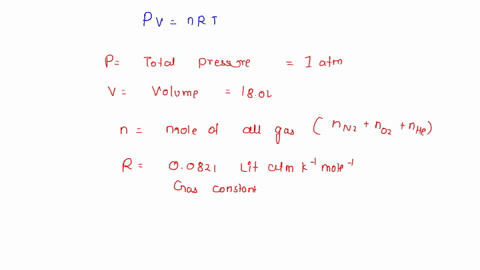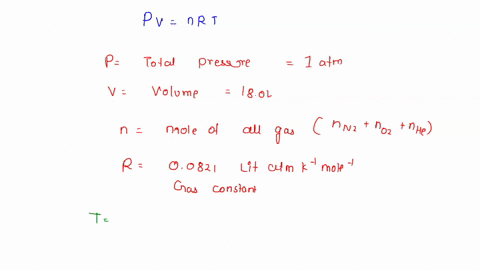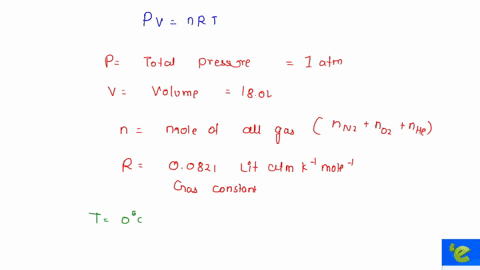
Solved To Use Partial Pressure In Gas Law Calculations In A Mixture Of Gases The Total Pressure Of The Gas Mixture Is Equal To The Sum Of The Partial Pressures Of The

Carbon Dioxide Wikipedia

Aleks Calculating Partial Pressure Of A Gas From A Sketch Youtube
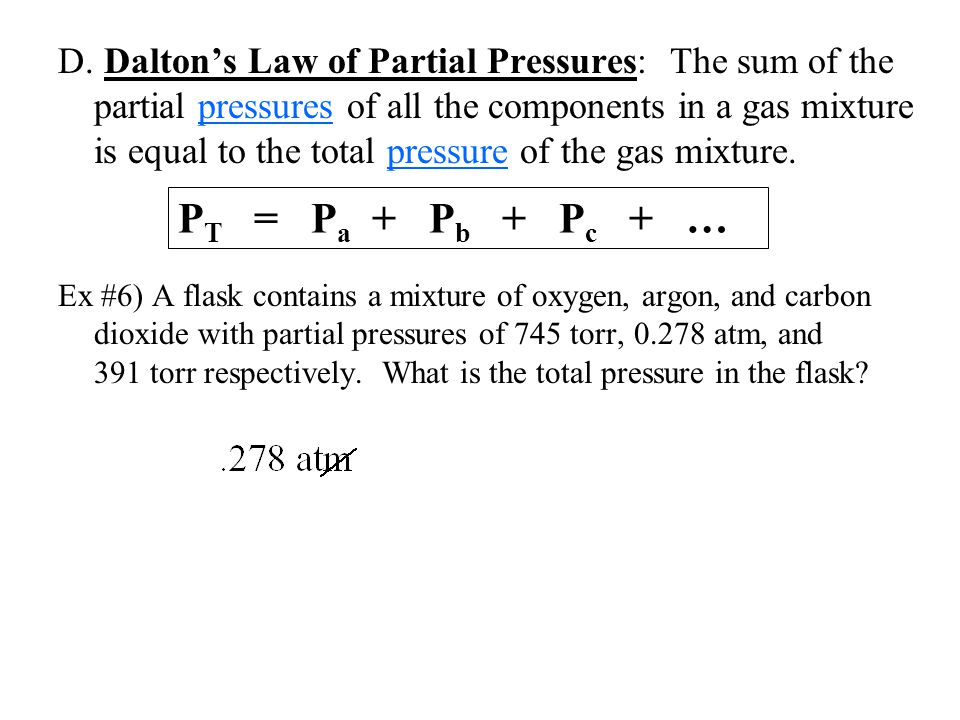
Gases Notes Ppt Video Online Download
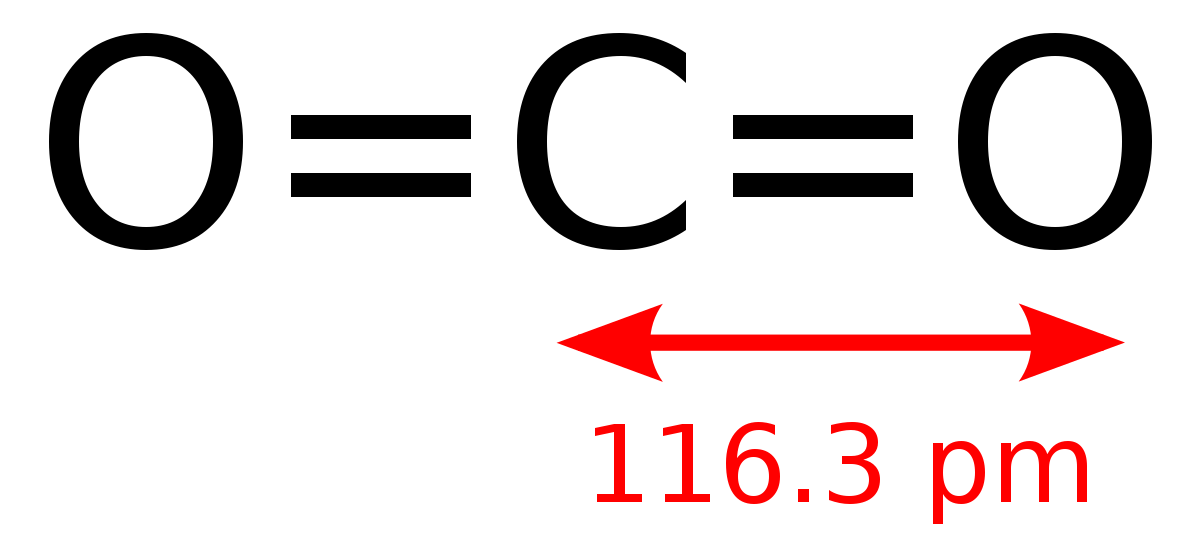
Carbon Dioxide Wikipedia

Chapter 6 Unit 4 Gas Laws Gsusurveychemistry Org
Carbon Dioxide Wikipedia

I Dalton S Law A The Total Pressure Of A Mixture Of Gases Equals The Sum Of The Pressures Each Gas Would Exert Independently 1 P Total P 1 P 2 Ppt Download
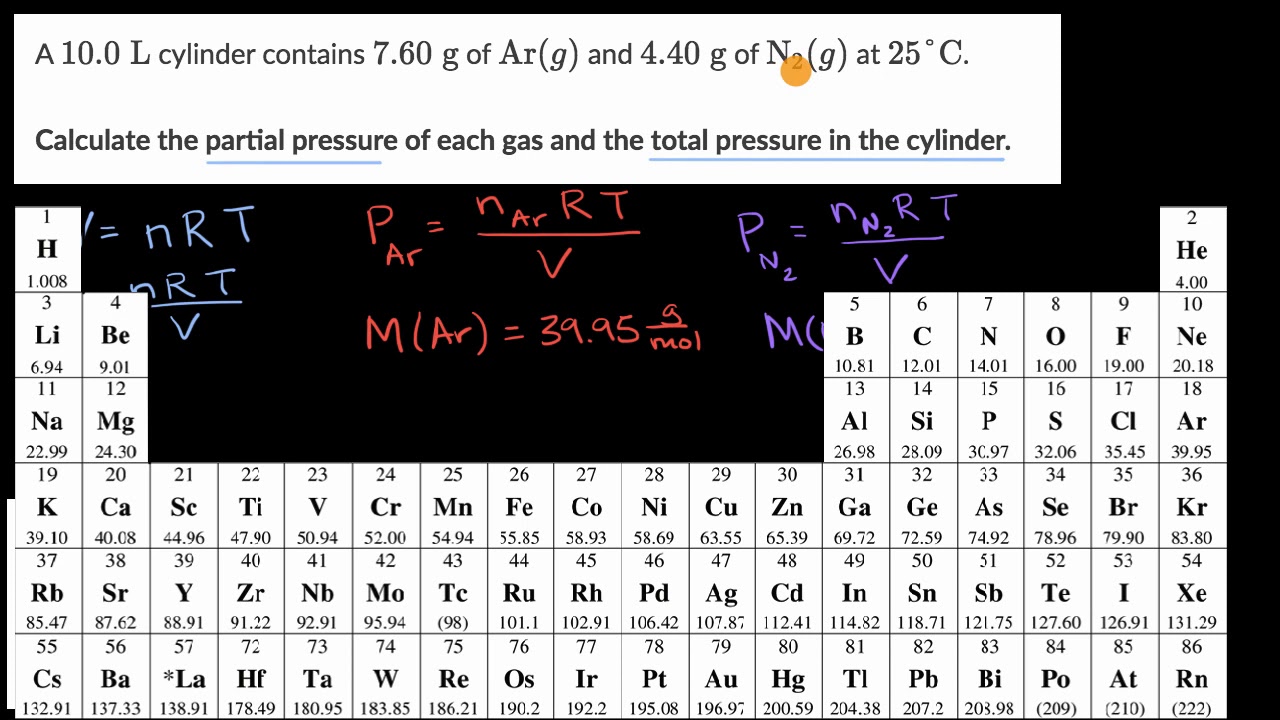
Worked Example Calculating Partial Pressures Ap Chemistry Khan Academy Youtube

Solved To Use Partial Pressure In Gas Law Calculations In A Mixture Of Gases The Total Pressure Of The Gas Mixture Is Equal To The Sum Of The Partial Pressures Of The
Mixtures Of Gases Partial Pressures

Aleks Calculating Partial Pressure In A Gas Mixture Youtube

The Combined And Ideal Gas Laws Ppt Download

Molecular Formulas And Nomenclature